Atomic Structure
 All elements are made up of atoms. All atoms are neutral.
All elements are made up of atoms. All atoms are neutral.
There are three particles in an atom. Positive protons, neutral neutrons and negative electrons.
The protons and neutrons make up the nucleus and the electrons surround the nucleus in shells (also called energy levels).
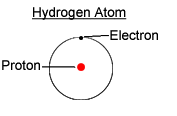
Hydrogen is the simplest element known. It contains one electron and one proton. It is the only atom to contain no neutrons.
Atoms of the same element have the same atomic number.
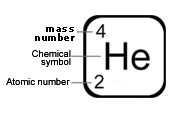 Atomic number = number of protons.
Atomic number = number of protons.
Atomic number = number of electrons.
Number of protons = number of electrons.
Mass number = number of protons + number of neutrons.
Mass number is measured in atomic mass units, u, and is always a whole number. Each proton or neutron is equal to 1 atomic mass unit.
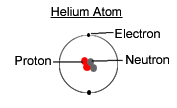
Helium has two protons and two electrons which give it a atomic number of 2. The two neutrons plus the two protons gives a mass number of 4.
An isotope is an atom with the same number of protons but different number of neutrons. Therefore the mass number changes but the atom remains neutral because neutrons have no charge.
Isotopes can be represented in several ways. For example, the isotope of chlorine with a mass number of 35 can be written as Chlorine-35 or ![]() . Below are the diagrams of other isotopes:
. Below are the diagrams of other isotopes:
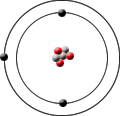 Lithium-6 has 3 protons,3 neutrons and 3 electrons.
Lithium-6 has 3 protons,3 neutrons and 3 electrons.
Lithium-7 has 3 protons, 4 neutrons and 3 electrons.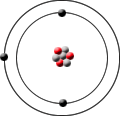
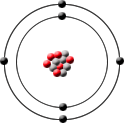 Carbon-12 has 6 protons, 6 neutrons and 6 electrons.
Carbon-12 has 6 protons, 6 neutrons and 6 electrons.
Carbon-13 has 6 proton, 7 neutrons and 6 electrons.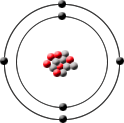
Chlorine has two isotopes: Chlorine-35 and Chlorine-37. Chlorine has an atomic number of 17. It has 17 protons and 17 electrons.
The number of neutrons = mass number - atomic number
Therefore:
Chlorine-35 isotope has 35-17=18 neutrons.
Chlorine-37 isotopes has 37-17=20 neutrons.
The mass of an atom in grams is so small it is too awkward to deal with so instead we use relative atomic mass, ![]() . The carbon-12 isotope is used as a reference and is given the value 12, all other atoms are measured relative to this. Relative atomic mass does not have any units and they are hardly ever whole numbers.
. The carbon-12 isotope is used as a reference and is given the value 12, all other atoms are measured relative to this. Relative atomic mass does not have any units and they are hardly ever whole numbers.
To work out the relative atomic mass of an element with isotopes can be worked out as follows:
Isotope number x %
The relative atomic mass of a particular atom can be considered an average of the mass number of the different isotopes. For example Chlorine has two isotopes: Chlorine-35 and Chlorine-37. The 37 Cl isotope only accounts for 25% of the atoms whereas the 35 Cl accounts for 75% of the atoms. So the relative atomic mass is worked out by multiplying the percentage prevalence by the isotope number and adding them together: (75/100 x 35) + (25/100 x 37) = 35.5.
Electrons orbit the atomic nucleus in shells (also called energy levels). For GCSE chemistry you need to know how the electrons fill the shells for the first 20 elements.
- The first shell can hold 2 electrons
- The second shell can hold 8 electrons
- The third shell can hold 8 electrons (This shell can actually hold 18 electrons but after 8 electrons a stability occurs with this shell and the fourth shell starts filling)
- The fourth shell can 2 electrons
| Element | No. of electrons |
Electron configuratioin |
| Hydrogen | 1 | 1 |
| Helium | 2 | 2 |
| Lithium | 3 | 2,1 |
| Beryllium | 4 | 2,2 |
| Boron | 5 | 2,3 |
| Carbon | 6 | 2,4 |
| Nitrogen | 7 | 2,5 |
| Oxygen | 8 | 2,6 |
| Fluorine | 9 | 2,7 |
| Neon | 10 | 2,8 |
| Element | No. of electrons |
Electron configuratioin |
| Na | 11 | 2,8,1 |
| Mg | 12 | 2,8,2 |
| Al | 13 | 2,8,3 |
| Si | 14 | 2,8,4 |
| P | 15 | 2,8,5 |
| S | 16 | 2,8,6 |
| Cl | 17 | 2,8,7 |
| Ar | 18 | 2,8,8 |
| K | 19 | 2,8,8,1 |
| Ca | 20 | 2,8,8,2 |
The elements shown in red have full shell of electrons, these are particularly stable elements and are known as the noble gases.